

It is mostly known for being inįire extinguishers back in the day. USES AND IMPORTANCE: Carbon Tetrachloride is a type of chemical compound that is found inĬleaning products, fire extinguishers and refrigerants. it became more popular to create Carbon Tetrachloride from Methane in the US in the 1950's because there is not an abundant supply of Carbon Disulfide. now a days it is made mostly from Methane. He made Carbon Tetrachloride in a reaction from Carbon Disulfide and Chlorine. He was a chemist named Henri Victor Regnault. HISTORY: It was created or made by a french man in 1839. It looks like a clear liquid with a foul odor to it.īOILING POINT: The boiling point for Carbon Tetrachloride is 76.7 degrees Celsius or about 170 degrees Fahrenheit.įREEZING POINT: The freezing point is -23 degrees Celsius or -9 degrees Fahrenheit.ĭENSITY: Its density in natural form is 1.5867 g cm−3 as a liquid. MOLAR MASS: The molar mass is 153.82 g/mol.

“Sodium chloride crystals” By Stanislav.nevyhosteny – Own work (CC BY-SA 4.FORMULA: It is an organic compound with the formula CCl(subscript) 4. “Carbon Tetrachloride” By H Padleckas, vectorized by MaxDZ8 – H Padleckas, vectorized by MaxDZ8, Public Domain) via Commons WikimediaĢ. What Is Sodium Chloride and How Is It Used? Retrieved October 14, 2020, Available here. The key difference between carbon tetrachloride and sodium chloride is that carbon tetrachloride is a covalent chemical compound whereas sodium chloride is an ionic chemical compound. Summary – Carbon Tetrachloride vs Sodium ChlorideĬarbon tetrachloride and sodium chloride are different from each other according to the chemical structure, properties and their applications.
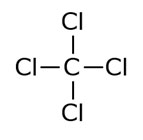
Moreover, carbon tetrachloride dissolves in nonpolar solvents while sodium chloride dissolves in polar solvents.īelow infograhic summarizes the differences between carbon tetrachloride and sodium chloride in tabular form. What is the Difference Between Carbon Tetrachloride and Sodium Chloride?īoth carbon tetrachloride and sodium chloride are chlorine-containing chemical compounds. In its pure form, sodium chloride cannot absorb water vapour, which means, it is not hygroscopic. At room temperature and pressure, sodium chloride appears in solid-state, as colourless crystals. The molar mass of this compound is 58.44 g/mol. Sodium chloride is an inorganic compound having the chemical formula NaCl. Moreover, we widely use it in fire extinguishers, as a precursor to refrigerants and as a cleaning agent. Once it was a popular solvent, but now we don’t use it due to its adverse health effects. Carbon tetrachloride is the key ingredient in lava lamps. Nowadays, we do not produce CFC since it harms the ozone layer. Before the prohibition, this compound was used to produce CFC in large scale. There are many uses of carbon tetrachloride. It resembles the structure of a methane molecule, which has four hydrogen atoms bonded to a single carbon atom. This geometry makes the compound nonpolar. Carbon tetrachloride is a known covalent compound where a carbon atom is covalently bonded to chlorine atoms. Therefore, we call it a “symmetrical geometry”. There are four chlorine atoms bonded to a single carbon atom, and the bond angles of the molecules are equal. When considering the geometry of the carbon tetrachloride molecule, it has the tetrahedral geometry. It has a melting point of −22.92 ☌, and the boiling point is 76.72 ☌. The molar mass of carbon tetrachloride is 153.81 g/mol. Figure 01: Structure of Carbon Tetrachloride


 0 kommentar(er)
0 kommentar(er)
